Is amount of heat required to raise material temperature by one unit.
Ceramic specific hear.
Steatite also known as soapstone or soaprock is a metamorphic rock a talc schist.
If we use the metric system the specific heat is the amount of heat that s needed for a sample which weighs 1 kg to elevate its temperature by 1k.
Thermodynamics effects of work heat and energy on systems.
Specific heat capacity is amount of heat required to raise temperature of unit mass of material by one unit.
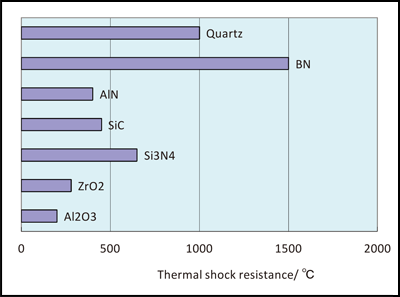
We have collected a number of charts detailing applications and properties for some of the most commonly used ceramic materials.
Of sic 2 3 ºfˉ 4 0 ºcˉ.
The effect of crystallization on the lattice vibrations of two glass ceramics a magnesium aluminosilicate corning code 9606 and a lithium aluminosilicate corning code 9623 is studied through measurements of the thermal conductivity and specific heat below 300 k.
M material mass.
δq amount of heat.
Heat capacity is amount of heat required to raise material temperature by one unit.
C δq mδt where c specific heat capacity.
Specific heat capacity of ceramic materials is higher than that of metals.
The specific heat of some commonly used solids is given in the table below.
Porcelain is a ceramic material made by heating selected and refined materials often including clay in the form of kaolinite to high temperatures.
δt temperature rise.
Specific heat capacity.
For conversion of units use the specific heat online unit converter.
Air specific heat at constant temperature and varying pressure figures and table showing isobaric cp and isochoric cv specific heat of air at constant temperature and.
This heat calculator or calorimetry calculator can help us determine the heat capacity of a sample that s heated or cooled.
Thermal expansion of ceramic materials is generally lower than that of metals.
Ceramic matrix composites prepregs for parts requiring thermal performance up to 2200 f.
Cordierite is a crystalline magnesium aluminosilicate.
Of al 13 ºfˉ 23 ºcˉ.
Material properties material properties for gases fluids and solids densities specific heats viscosities and more.